Understanding Chemical Bonds and Molecular Geometry
Chemical bonds are the forces that hold atoms together, with valence electrons playing a crucial role. Ionic bonds involve complete electron transfer between metals and nonmetals, while covalent bonds see electrons being shared. Lewis dot diagrams help in visualizing the valence electrons of atoms, aiding in understanding bonding patterns like paired and unpaired electrons. Octet rule states that most atoms aim for 8 electrons in their outer shell for stability.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
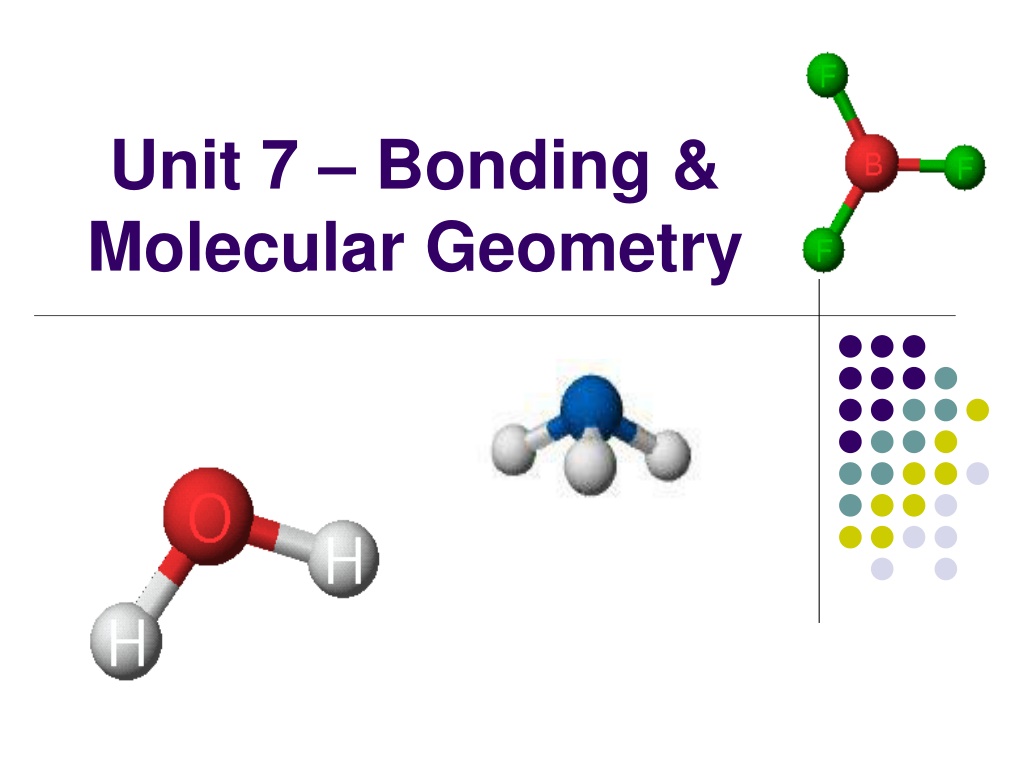
Unit 7 Bonding & Molecular Geometry
Definitions Chemical Bonds Force that holds atoms together It s all about the electrons (e-) Electrons available for bonding are called valence electrons!
Two Types of Chemical Bonds 1. Ionic Bonds Bond between metal and nonmetal due to electrostatic interactions Electrons are completely transferred from metal to nonmetal 2. Covalent Bonds Bonds in which electrons are shared
Definitions: (Ionic/Covalent Bonding) Octet rule (Rule of 8) Most atoms want 8 e- in the outer shell this is most stable H2and He want a duet (2 e-) Electron configuration for duet = ns2 Electron configuration for octet = ns2np6
Lewis Structures (Atoms) A Lewis dot diagram depicts an atom as its symbol and its valence electrons. Ex: Carbon C.. . Carbon has four electrons in its valence shell (carbon is in group 14), so we place four dots representing those four valence electrons around the symbol for carbon. .
Drawing Lewis Dot Diagrams Electrons are placed one at a time in a clockwise manner around the symbol in the north, east, south and west positions, only doubling up if there are five or more valence electrons. Same group # = Same Lewis Dot structure Ex. F, Cl, Br, I, At Cl. . . . .. . Example: Chlorine (7 valence electrons b/c it is in group 17)
Paired and Unpaired Electrons As we can see from the chlorine example, there are six electrons that are paired up and one that is unpaired. When it comes to bonding, atoms tend to pair up unpaired electrons. A bond that forms when one atom gives an unpaired electron to another atom is called an ionic bond. A bond that forms when atoms share unpaired electrons between each other is called a covalent bond.
Note: In the final structure, the placement of the dots around the element is not crucial: Maximum # of valence electrons = 8
Bonding in Ionic Compounds The ionic bond forms from attraction of cations for anions.
Structure of Ionic Compounds Ionic compounds have formula units these show ratio of ions in the crystal lattice.
Writing Lewis Structures for Ions Uses either 0 or 8 dots, brackets and a superscript charge designate to ionic charge Ex.) Li+, Be+2, B+3, C+4, N-3, O-2, F-1
Writing Lewis Structures (Ionic Compounds) Lewis Dot Diagrams of Ionic Compounds Ex. 1) MgO Ex. 2) Li2O
Drawing Lewis Structures (Covalent Compounds) Covalent compound - a substance made up of atoms which are held together by covalent bonds (in which e- are shared) They are also called molecules.
Covalent Compounds and Lewis Structures Lewis structures for covalent compounds show the bonds and how the atoms will connect. Shared e- = bonding e- Bonding pair generally shown as a straight line between atoms Non-shared e- = lone pair e- (a.k.a. non- bonding e-) Ex. H2O
Types of Covalent Bonds Single Bond 2 e- are shared in a bond (1 from each atom) Double Bond 2 pairs of e- are shared (4 e- total, 2 from each atom) Triple Bond 3 pairs of e- are shared (6 e- total, 3 from each atom)
Rules for Drawing Lewis Dot Diagrams 1. Add up the total number of valence e- for each atom in the molecule. Each (-) sign counts as 1 e-, each (+) sign subtracts one e- 2. Write the symbol for the central atom then use one pair of e- to form bonds between the central atom and the remaining atoms. 3. Count the number of e- remaining and distribute according to octet rule (or the duet rule for hydrogen) 4. If there are not enough pairs, make sure the most electronegative elements are satisfied. Then, start shifting pairs into double and triple bonds to satisfy the octet rule. 5. If there are extra e-, stick them on the central atom.
Hints: H is NEVER a central atom! Halogens (Group 17) are usually not central atoms. If you only have 1 of a certain element, it is usually the central atom.
Checking Your Work! But Remember.... The Structure MUST Have: the right number of atoms for each element, the right number of electrons, the right overall charge, and 8 electrons around each atom (ideally).
Examples: F2 H2O OCl- PO43-
Examples: O2 CH4 HF NH3
Examples: NH4+ SO32- N2 CH3OH
Exceptions to the Octet Rule Reduced Octets electron deficient molecules (Be and B) Be: 2 valence e-, doesn t form octet (BeH2: Be has 4 e-) BF3 B: 3 valence e-, doesn t form octet (BF3: B has 6 e-)
Exceptions to the Octet Rule Expanded Octets (Examples: P, S, Cl, As, Se, Br, Kr, Xe) How to recognize: The central atom in PERIOD 3 or greater is surrounded by > 4 atoms. You draw the Lewis diagram and the results don t make sense the central atom has > 8 e- 1. 2.
Expanded Octets (P, S, Cl, As, Se, Br, Kr, Xe) Examples: PF5 XeF4
Resonance Structures Definition: When a single Lewis structure does not adequately represent a substance, the true structure is intermediate between two or more structures which are called resonance structures. Resonance Structures are created by moving electrons, NOT atoms.
Resonance Structure Example, SO2 Central atom = S This leads to the following structures: These equivalent structures are called RESONANCE STRUCTURES. The true structure is a HYBRID of the two. Arrow means in resonance with
Resonance Structure Example, NO3- Draw the Lewis diagram for NO3- with all possible resonance structures.
Free Radicals When there is an odd # of total electrons, there will be a single, unpaired electron in the structure! Example: NO Radicals are extremely reactive; they want to have paired electrons to be more stable!!
Molecular Geometry Molecular Geometry describes the 3-D arrangement of atoms in a molecule. We will use VSEPR theory to predict these 3-D shapes!
There is a fundamental geometry that corresponds to the total number of electron pairs around the central atom: 2, 3, 4, 5 and 6 linear trigonal planar tetrahedral trigonal bipyramidal octahedral
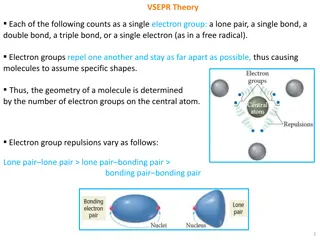
VSEPR: Shapes of Molecules VSEPR Theory (definition) Valence Shell Electron Pair Repulsion Based on idea that e- pairs want to be as far apart as possible The molecule adopts the shape that minimizes the electron pair repulsions. Based on molecular shape of Lewis structure
VSEPR: Shapes of Molecules We define the electron pair geometry by the positions in 3D space of ALL electron pairs (bonding and non-bonding). The molecular geometry only considers the positions of the bonded electrons.
To determine the electron pair geometry: 1. Draw the Lewis structure. 2. Count the number of bonded (X) atoms and non-bonded or lone pairs (E) around the central atom. 3. Based on the total of X + E, assign the electron pair geometry. 4. Multiple bonds count as one bonded pair!
Basic Electron Pair Geometries Shapes 1. Linear Sum of Bonded Atoms & Lone e- 2 2. Trigonal planar 3 3. Tetrahedral 4 4. Trigonal bipyramidal 5 5. Octahedral 6
Molecular Geometry Notation A: Central Atom X: Bonded Atom E: Non-bonding electron pair (Lone pair e- on central atom) BP s: Bonding Pairs LP s: Lone Pairs
Molecular Geometry: Two Bonded Items Electron Pair Geometry = linear # Total Bonds to C.A. # lone pairs (E) bonded atoms (X) AXE Notation Molecular Geometry Bond Angles Example 2 0 AX2 180 2 Linear BeCl2 X A X
Molecular Geometry: Three Bonded Items Electron Pair Geometry = trigonal planar # Total Bonds to C.A. # lone pairs (E) bonded atoms (X) AXE Notation Molecular Geometry Bond Angles Example Trigonal Planar 3 0 AX3 120 BCl3 3 118 2 1 AX2E Bent NO2-
Molecular Geometry: Four Bonded Items e- pair geometry = tetrahedral X A X X X Total Bonds to C.A. Bonded atoms (X) Lone pairs (E) AXE Notation Molecular Geometry Bond AnglesExample 4 0 AX4 CCl4 Tetrahedral 109.5 Trigonal pyramid 4 3 1 AX3E NH3 107 2 2 AX2E2 Bent H2O 104.5
Total Bonds to C.A. Bonded atoms (X) Lone pairs (E) AXE Notation Molecular Geometry Bond AnglesExample 90, 180, 120 <90, 180, 120 <90, 180 Trigonal bipyramid 5 0 AX5 AsCl5 4 1 AX4E SeCl4 See-saw 5 3 2 AX3E2 BrCl3 T-shaped 2 3 AX2E3 Linear XeF2 180 X X X A X X
AX5 AX4E AX3E2 AX2E3 Molecular Geometries for Five Electron Pairs All based on trigonal bipyramid!
Molecular Geometry:6 Bonded Items Total Bonds to C.A. Bonded atoms (X) Lone pairs (E) AXE Notation Molecular Geometry Bond AnglesExample 90, 180 <90, 180 6 0 AX6 TeBr6 Octahedral Square Pyramid Square Planar 5 1 AX5E BrF5 6 4 2 AX4E2 XeF4 <90 3 3 AX3E3 --- T-shaped <90 2 4 AX2E4 Linear --- 180
Molecular Geometry: Six Bonded Items X X X A X X X AX6 AX5E AX4E2
Electronic Flashcards http://www.proprofs.com/flashcards/cards.php?i d=7521 Flash cards on molecular geometry and hybridization
Molecules with More than One Central Atom Determine geometry for each central atom separately! Example: In acetic acid, CH3COOH, there are three central atoms:
Molecules with only two atoms are always linear! Examples: HCl N2
What shape (molecular geometry) would the following compounds have according to VSEPR theory? O2 CFCl3
What shape (molecular geometry) would the following compounds have according to VSEPR theory? H2S PBr5
What shape (molecular geometry) would the following compounds have according to VSEPR theory? SeCl22- C2H2 (hint: classify each C separately)