Point-of-Care Molecular Diagnostics Market
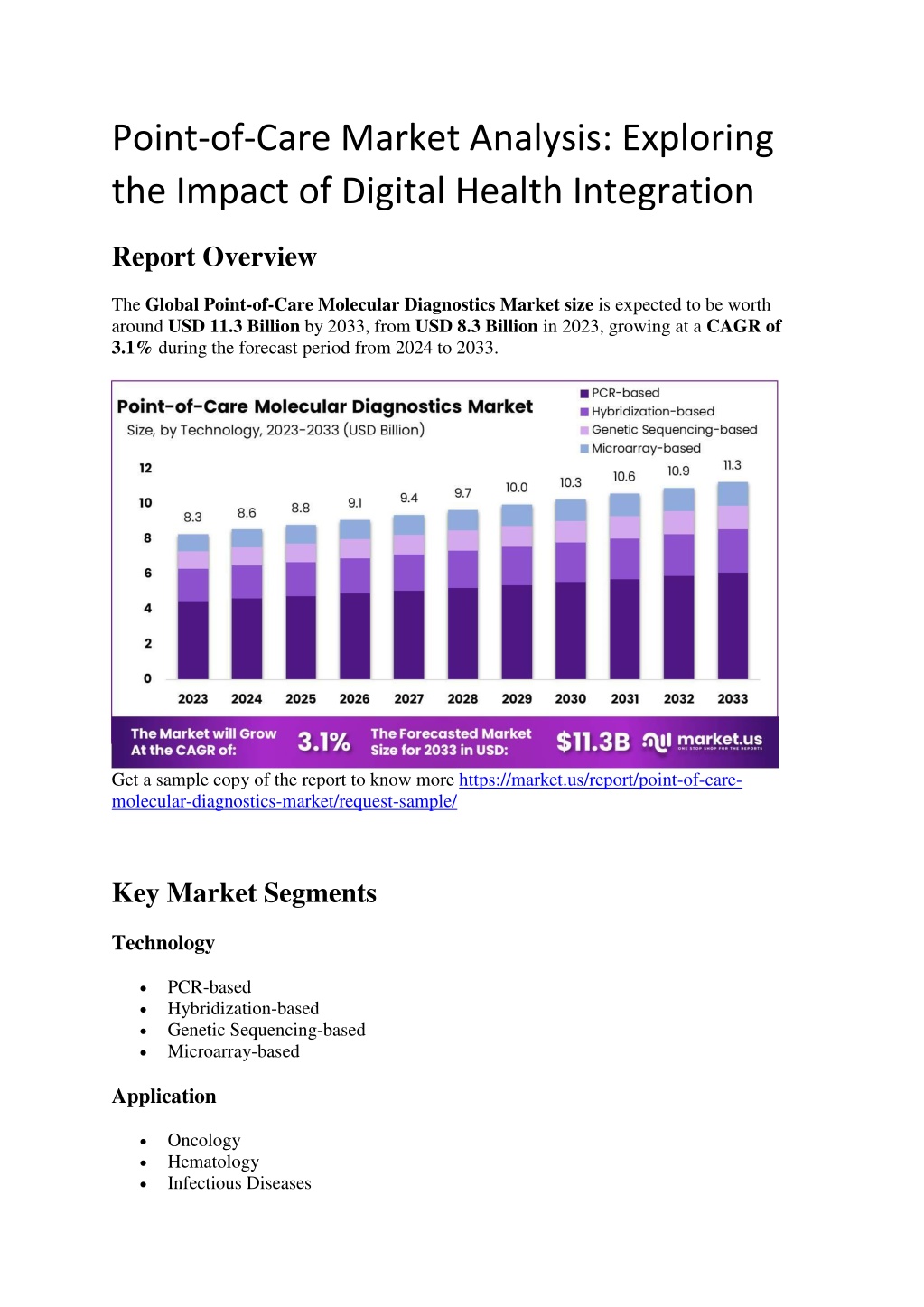
The Global Point-of-Care Molecular Diagnostics Market size is expected to be worth around USD 11.3 Billion by 2033, from USD 8.3 Billion in 2023, growing at a CAGR of 3.1% during the forecast period from 2024 to 2033.nn
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
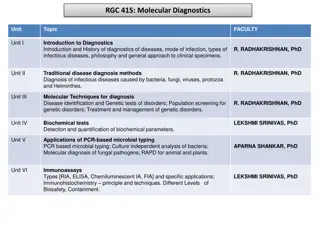
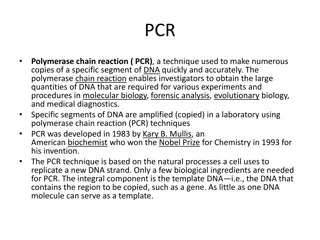
Point-of-Care Market Analysis: Exploring the Impact of Digital Health Integration Report Overview The Global Point-of-Care Molecular Diagnostics Market size is expected to be worth around USD 11.3 Billion by 2033, from USD 8.3 Billion in 2023, growing at a CAGR of 3.1% during the forecast period from 2024 to 2033. Get a sample copy of the report to know more https://market.us/report/point-of-care- molecular-diagnostics-market/request-sample/ Key Market Segments Technology PCR-based Hybridization-based Genetic Sequencing-based Microarray-based Application Oncology Hematology Infectious Diseases
Prenatal Testing Other Applications Test Location OTC POC End-use Hospitals Home-care Decentralized Labs Research Institutes Other End-Uses Key Regions North America (The US, Canada, Mexico) Western Europe (Germany, France, The UK, Spain, Italy, Portugal, Ireland, Austria, Switzerland, Benelux, Nordic, Rest of Western Europe) Eastern Europe (Russia, Poland, The Czech Republic, Greece, Rest of Eastern Europe) APAC (China, Japan, South Korea, India, Australia & New Zealand, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam, Rest of APAC) Latin America (Brazil, Colombia, Chile, Argentina, Costa Rica, Rest of Latin America) Middle East & Africa (Algeria, Egypt, Israel, Kuwait, Nigeria, Saudi Arabia, South Africa, Turkey, United Arab Emirates, Rest of MEA) Market Key Players F. Hoffmann-La Roche AG Abbott Laboratories QIAGEN AV Bayer AG Nova Biomedical Danaher Nipro Diagnostics Bio-Rad Laboratories Inc. Agilent Technologies Inc. bioM rieux OraSure Technologies If You Have Any Questions About This Report, Please Reach Out to Us @ https://market.us/report/point-of-care-molecular-diagnostics-market/#inquiry Drivers
1.Rising Demand for Early Disease Detection: The need for timely and accurate disease diagnosis is increasing. Point-of-care molecular diagnostics offer rapid results, driving market growth. 2.Technological Advancements: Innovations in molecular diagnostics technology are enhancing the efficiency and accuracy of point-of-care testing. This boosts market expansion. 3.Increase in Chronic Diseases: The prevalence of chronic conditions such as diabetes and cardiovascular diseases drives the demand for quick diagnostic solutions. 4.Growing Geriatric Population: The aging population is more prone to chronic diseases, leading to a higher demand for point-of-care diagnostics. 5.Enhanced Patient Convenience: Patients prefer point-of-care tests for their convenience and speed, which is fueling market growth. 6.Cost-Effective Solutions: Point-of-care diagnostics can reduce overall healthcare costs by providing immediate results and reducing the need for further testing. 7.Improved Healthcare Infrastructure: Expansion of healthcare infrastructure, especially in remote areas, supports the adoption of point-of-care molecular diagnostics. Trends 1.Integration of AI and Machine Learning: AI and machine learning are being integrated into molecular diagnostics, improving test accuracy and decision-making. 2.Increased Focus on Infectious Diseases: There is a growing trend towards developing point-of-care diagnostics for infectious diseases like COVID-19 and influenza. 3.Shift Towards Personalized Medicine: Personalized medicine is driving the demand for point-of-care diagnostics tailored to individual patient needs. 4.Adoption of Portable Devices: The market is witnessing a rise in portable point-of- care diagnostic devices, making testing more accessible. 5.Expanding Applications: Point-of-care molecular diagnostics are expanding beyond traditional uses to include areas like oncology and genetic testing. 6.Enhanced Regulatory Approvals: Regulatory bodies are streamlining the approval processes for point-of-care diagnostic devices, encouraging innovation and market entry. 7.Growing Consumer Health Awareness: Increased health awareness among consumers is driving demand for accessible and efficient diagnostic solutions. Opportunities 1.Emerging Markets: Expanding healthcare markets in developing countries present significant growth opportunities for point-of-care molecular diagnostics. 2.Partnerships and Collaborations: Strategic partnerships between diagnostic companies and healthcare providers can drive market innovation and growth. 3.Expansion in Home Diagnostics: The trend towards home-based diagnostic solutions opens new avenues for point-of-care molecular diagnostics. 4.Development of Novel Biomarkers: Research into new biomarkers can lead to advanced diagnostic tests, creating new market opportunities. 5.Government Initiatives: Government programs aimed at improving healthcare access and affordability can boost market growth.
6.Increased Investment in R&D: Investment in research and development is expected to drive innovation and introduce advanced point-of-care diagnostics. 7.Integration with Digital Health Platforms: Combining point-of-care diagnostics with digital health platforms offers new opportunities for data management and patient engagement. Restraints 1.High Initial Costs: The high cost of advanced point-of-care molecular diagnostic devices can be a barrier to market adoption. 2.Regulatory Challenges: Stringent regulatory requirements can delay the approval and commercialization of new diagnostic products. 3.Limited Reimbursement: Inadequate reimbursement policies can impact the affordability and accessibility of point-of-care molecular diagnostics. 4.Technical Complexities: The complexity of molecular diagnostic technologies can limit their use in low-resource settings. 5.Data Privacy Concerns: Issues related to data privacy and security can hinder the adoption of point-of-care diagnostics integrated with digital platforms. 6.Competition from Traditional Diagnostics: Traditional diagnostic methods remain widely used and can compete with emerging point-of-care technologies. 7.Lack of Skilled Personnel: The limited availability of trained healthcare professionals can affect the implementation and effective use of point-of-care molecular diagnostics. Contact Us : 420 Lexington Avenue, Suite 300 New York City, NY 10170, United States Phone:+1 718 618 4351 (International),+91 78878 22626 (Asia) Email: inquiry@market.us